The Challenge:
Recent advancements in immuno-oncology and CAR-T cell therapies have led to significant changes in the standard of care of patients with cancer but these benefits remain accessible to only a fraction of individuals. Current cellular therapies, proven effective in B cell malignancies, exhibit only modest gains against solid tumors, and many carry the risk of severe adverse effects that handicap the ability sustain a response or engender combination strategies.
Mongoose Bio is advancing a therapeutic approach that will fulfill the promise of T cell therapy for patients with solid tumors by addressing two of the major challenges limiting the efficacy and broader applicability of conventional T cell therapy:
2. Limited persistence of transferred T cells to sustain a clinical response and provide long term immunoprotection.
The Solution:
Mongoose Bio addresses both major challenges by advancing an approach based on TCRs directed against important immunogenic pan-cancer antigens and a memory programming module that will confer in vivo longevity to transferred T cells.
1. Novel targets for TCR-based cell therapy:
TCR-T therapies utilize the T cell receptor (TCR) to engage tumor targets. Because TCRs recognize surface MHC presented fragments of protein (peptides) that can originate from any cellular compartment, TCR targets can be a transcription factor, oncogene, cancer-testis antigen, DNA repair gene, chemoresistance gene or any of the thousands of tumor-associated proteins found in solid tumors. Mongoose Bio has interrogated its database of peptide target spectra and we have selected the highest-ranking antigens for our drug development portfolio.
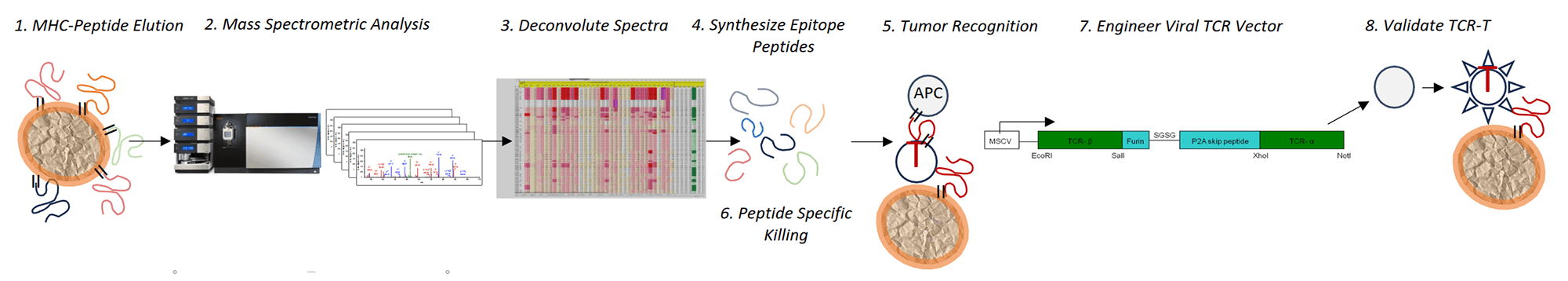
The Tumor Immunopeptidome Discovery Platform
2. T Cell Persistence:
The second major challenge to effective T cell therapy has always been the limited persistence in vivo of the adoptively transferred T cells, leading to relapse rates as high as 60% despite patients achieving complete remission following CAR-T cell therapy. In large part, this is attributable to the absence of “T cell memory” in the transferred cells – a feature absent from almost every cell therapy modality to date.
Clinical Evidence shows Epigenetic Tcm Reprogramming Prevents Recurrence of High-risk AML
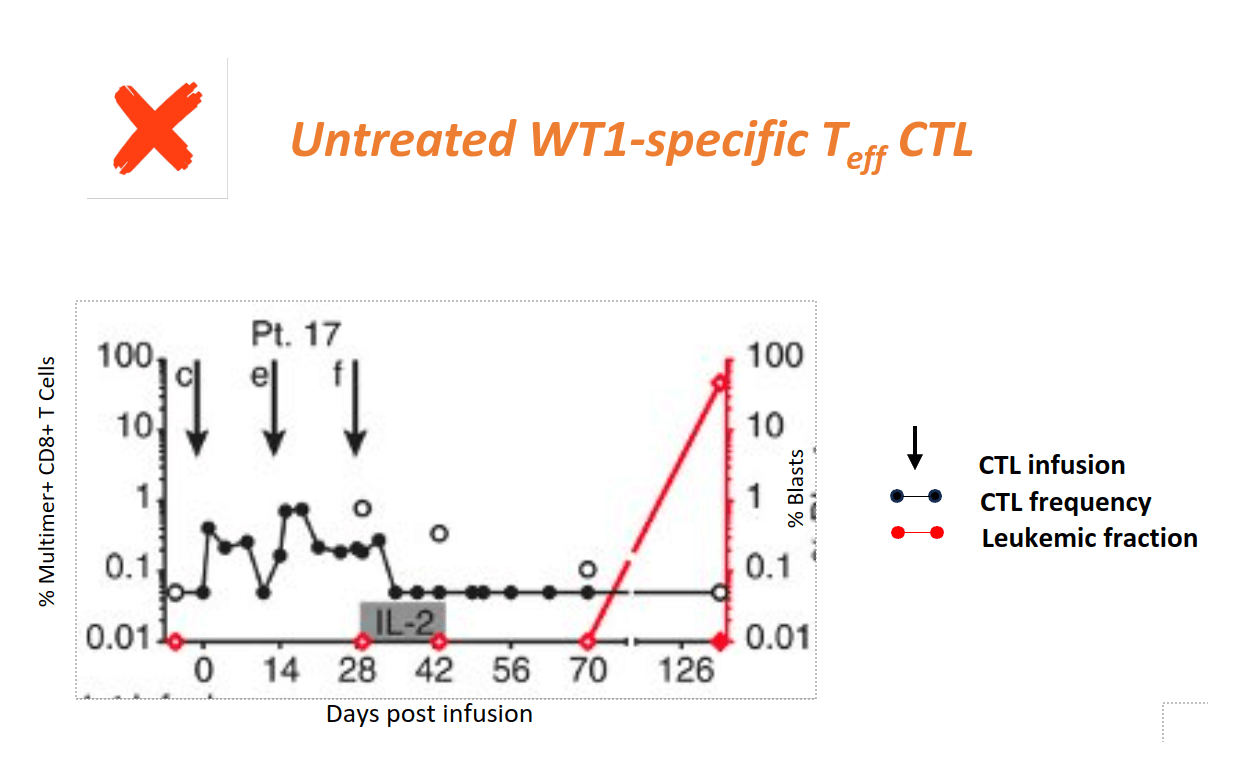
Patients receiving Teff CTL for high-risk AML relapse when WT1-CTL do not persist
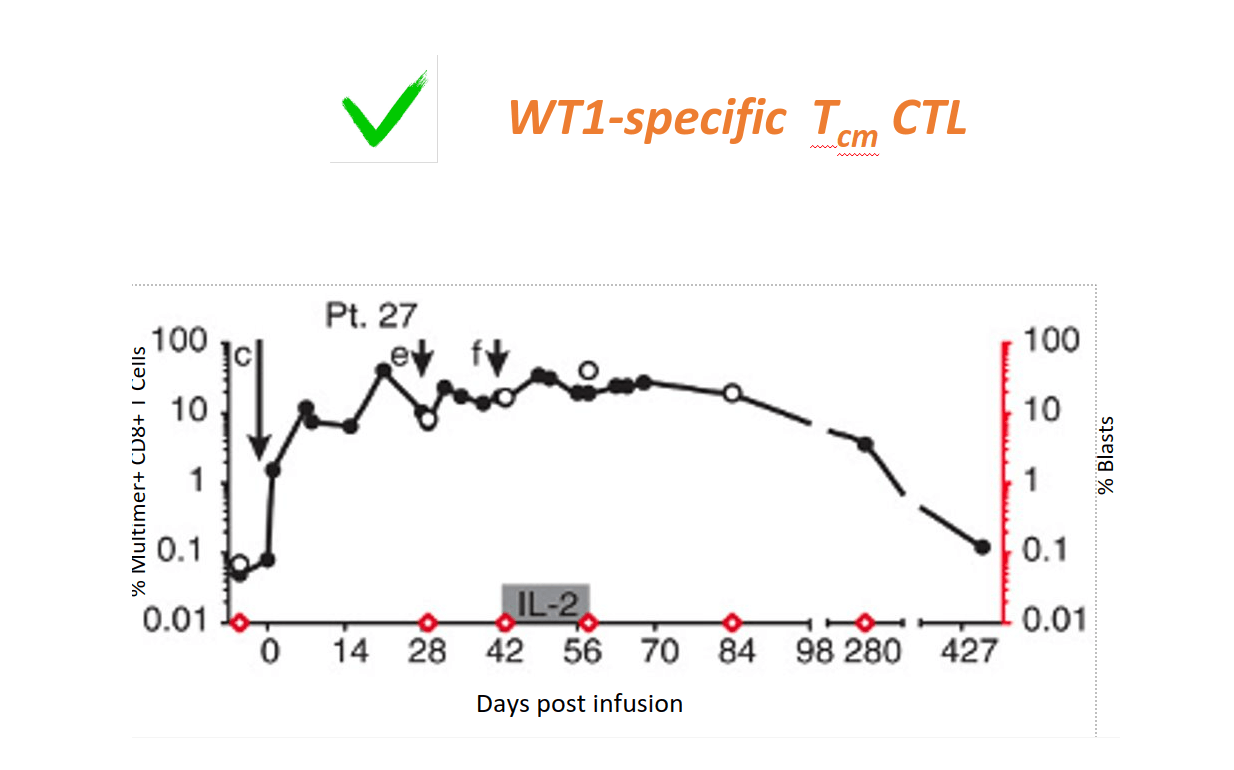
Patients receiving WT-1 specific Tcm CTL remain in CR; WT1-specific CTL persist
Epigenetic Reprogramming leads to Persistence of T cells and Clinical Benefit
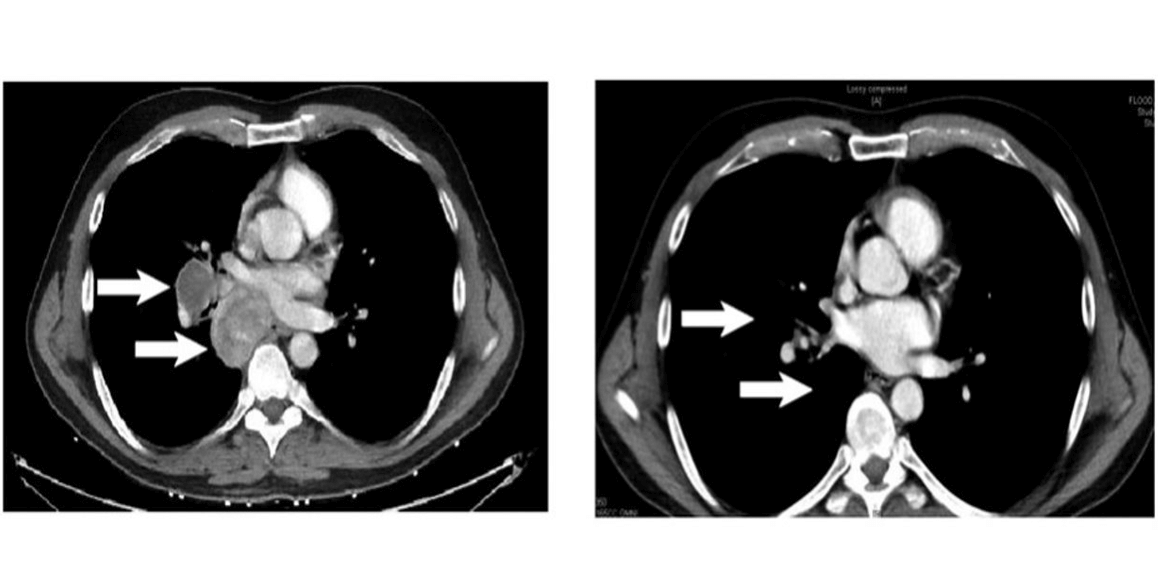
Patient failing anti CTLA4 monotherapy, Rx with MART-1 specific Tcm + ICI complete → response of intrathoracic metastases
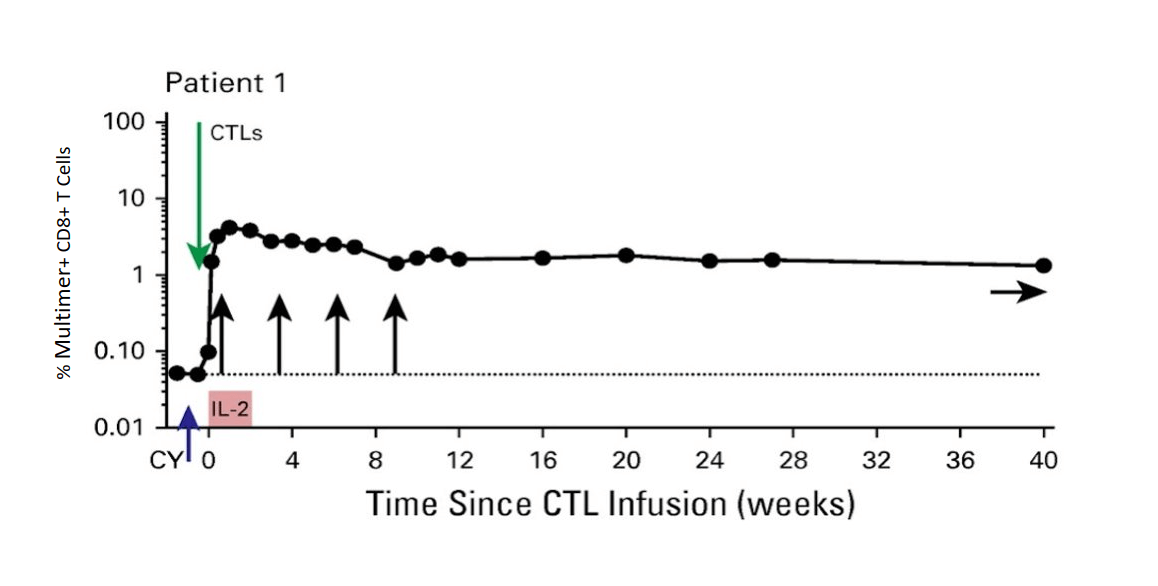
MART-1 specific Tcm persist long term (> 40 weeks)
Differentiation:
- Highly Immunogenic Tumor Selective Native T Cell Receptor Targets
- Immunopeptidome Discovery Platform identifies and characterizes bona fide MHC-presented tumor derived peptides
- 250 high value TCR target antigens have been empirically validated with an ex vivo workflow
- 4 top scoring targets that cover > 80% of Class I MHC (HLA) already selected for the pipeline
- Immunopeptidome Discovery Platform identifies and characterizes bona fide MHC-presented tumor derived peptides
- Native TCR Isolation Precludes Synthetic TCR Binding Optimization or Inappropriate CD28- / 41BB Signaling
- High affinity MHC-restricted target specific patient derived CTL’s identified
- Bespoke TCRa & TCRβ chains sequenced and expressed in a characterized retroviral vector
- Clinically Proven Epigenetic Central Memory T Cell Reprogramming
- IL-21 and HDACi based protocols drive T cells into central memory state
- Patients receiving target specific Tcm demonstrate complete and durable responses in clinical trials
- Autologous CTL-T Therapy Does Not Require Aggressive Lymphodepletion
- Several first-in-human clinical studies demonstrate long-term in vivo persistence of transferred central memory CD8+ T cells without any requirement for high dose lymphodepletion
- Favors therapy shaping by combinations with immuno-modulators (ex. anti-CTLA4, anti-PD1/PD-L1)
Lead asset: MGB-001
Our MGB-001 TCR-T is a high-affinity T cell receptor engineered T cell. It was sourced from T cells created using a highly immunogenic HLA-A2-restricted epitope identified by a proprietary mass spectrophotometry (MS)-based immunopeptidome discovery platform (IDP). Unlike other TCRs on the market, ID/validation of this TCR epitope was rigorously selected from among an unbiased pool of 1000s of well-curated MHC-eluted peptides, empirically validated, and clinically annotated to target pan-cancers. Our product is highly immunogenic, targets a protein broadly expressed by many solid tumors, and addresses HLA subtypes representing at least 60% of the global patient population in common cancers. Importantly, there is no off-target activity due to high specificity for the expected target tumor cells – expression of our target (MGB-001) is not seen in normal cells (germinal tissues only).
Lead Asset: MGB-001
- A classic cancer-testis antigen
- Expressed in over 60% of breast cancers
- Expressed in approximately 45% of prostate, lung, bladder, gastric, ovarian, head/neck cancers
- Presented by both HLA-A2 and A3/A11 subtypes representing >60% of all Western & Asian cancer patients
- No previously known TCR targets
- Highly tumor selective and tumorigenic
- Associated with epithelial mesenchymal transformation (EMT) and DNA repair following radiation and chemotherapy
- Expressed at high levels across multiple common and rare cancers
Pipeline
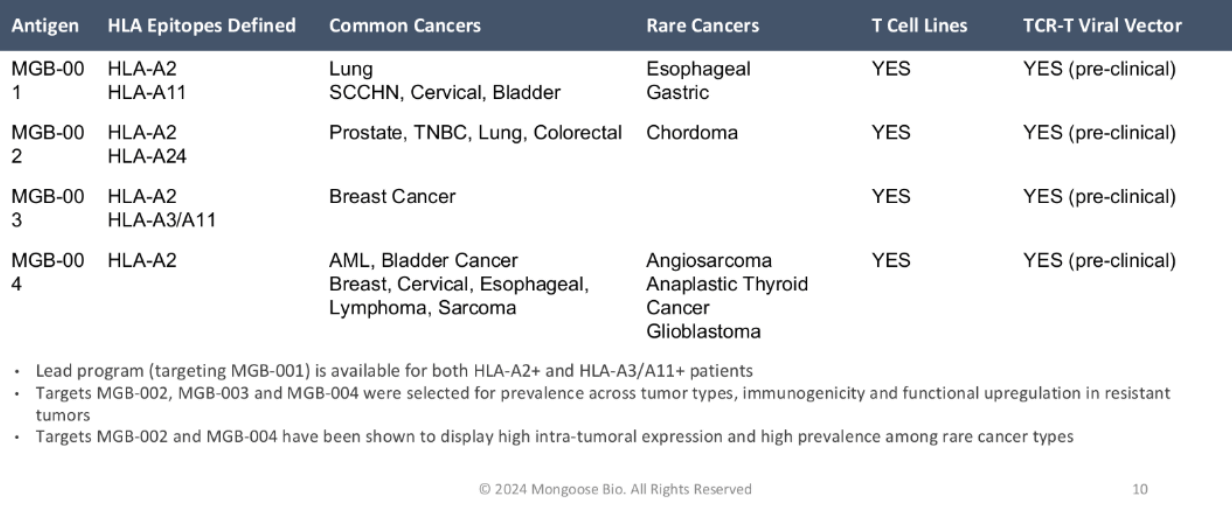
| Antigen | HLA Epitopes Defined | Common Cancers | Rare Cancers | T Cell Lines | TCR-T Viral Vector |
| MGB-001 | HLA-A2 HLA-A11 |
Lung HNSCC, Cervical, Bladder |
Esophageal Gastric |
YES | YES (pre-clinical) |
| MGB-002 | HLA-A2 HLA-A24 |
Prostate, TNBC, Lung, Colorectal | Chordoma | YES | YES (pre-clinical) |
| MGB-003 | HLA-A2 HLA-A3/A11 |
Breast Cancer | YES | YES (pre-clinical) | |
| MGB-004 | HLA-A2 | AML, Bladder Cancer Breast, Cervical, Esophageal, Lymphoma, Sarcoma |
Angiosarcoma Anaplastic Thyroid Cancer Glioblastoma |
YES | YES (pre-clinical) |